 |
 |
 |
Physical background of our research
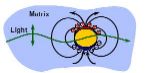 Gold and silver nanoparticles exhibit interesting color effects in the visible spectral range, which is the resonant excitation of a coherent oscillation of the conduction band electrons at a certain frequency or color. This causes the color to be dependent color on the shape and size of the particle.
Gold and silver nanoparticles exhibit interesting color effects in the visible spectral range, which is the resonant excitation of a coherent oscillation of the conduction band electrons at a certain frequency or color. This causes the color to be dependent color on the shape and size of the particle. In suspensions, for example, gold nanoparticles appear red in contrast to the usual yellow shimmer. With partial aggregation, the color changes more strongly to dark blue and purple. These phenomena have been known for a long time and the principal has been theoretically understood for over 100 years see History). We use these properties in a novel way to explore single biological molecules.
In suspensions, for example, gold nanoparticles appear red in contrast to the usual yellow shimmer. With partial aggregation, the color changes more strongly to dark blue and purple. These phenomena have been known for a long time and the principal has been theoretically understood for over 100 years see History). We use these properties in a novel way to explore single biological molecules.
Background
Metal nanoparticles, especially those made of gold and silver, exhibit strong absorption in the visible, near-infrared spectral range, which is the result of plasmon excitation. Plasmons are collective coherent oscillations of the conduction band electrons (the so-called electron gas or plasma) against the positively charged ionic lattice. The natural oscillation of this mode in gold can be excited by light with a wavelength of about 530 nm, in silver at about 400 nm. The resonance wavelength depends on the sorroundings of the particle and especially on the shape. By synthesizing particles of appropriate shapes, the resonance wavelength can be tuned to a wide spectral range, even to infrared. The excitation manifests itself in an increased absorption and scattering at the corresponding wavelength, many orders higher than for particles of the same size and shape of other materials. The absorption cross-section can even be higher than the geometrical cross-section. This thousand-fold increased absorption at the plasmon wavelength leads to an intensive color of even very diluted colloidal gold and silver particle solutions. This special property has long been employed in the coloring of glass and is currently being discussed for a number of other applications such as amplification of non-linear optical effects, for example, of Raman scattering, the sensitive detection of biological molecules, and markers in experiments on single biological molecules.
Our measuring principle
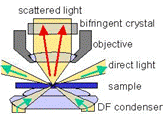
... is dark field microscopy using white light illumination. The light passes (green arrows) by the objective of the microscope, except for the scattered part which finds its way into the objective. Thus, the particles are seen in bright light in front of a dark background (hence the name dark field) in high contrast (larger than 1:1 000 000).  This measuring principle requires a great amount of carefulness in its execution because any distortion that could come from dust particles has to be avoided. In the animated picture on the left, you can see the scattered light of small gold particles (about 40 nm) in an aqueous solution. The movement of the particles is due to Brownian motion. Some particles adhere to the glass surface of the probe chamber and don't move. The movement is shown in real-time and the contrast and brightness match the natural perception of the naked eye.
This measuring principle requires a great amount of carefulness in its execution because any distortion that could come from dust particles has to be avoided. In the animated picture on the left, you can see the scattered light of small gold particles (about 40 nm) in an aqueous solution. The movement of the particles is due to Brownian motion. Some particles adhere to the glass surface of the probe chamber and don't move. The movement is shown in real-time and the contrast and brightness match the natural perception of the naked eye.
Gold nanorods as orientation sensors
 Rod shaped gold nanoparticles exhibit especially interesting properties, shown in the picture on the right, which was taken with a transmission electron microscope. These nanoparticles are 20 nm wide and 60 nm long. Due to their shape, the scattered light is strongly polarized along the particle axis. This can be used to manufacture polarizers for example. Another very interesting application is the use of the polarisation as a nanosensor for orientation, which is a property that is otherwise very hard or even impossible to obtain on a nanometer scale. Thus, we are planning to use such rods to explore single molecules such as molecular motors. The measuring principle is depicted in the picture on the right: the relative orientation is determined by comparing the intensities of the two points. The points are created by light passing through a birefringent crystal. More details can be found in this publication:
Rod shaped gold nanoparticles exhibit especially interesting properties, shown in the picture on the right, which was taken with a transmission electron microscope. These nanoparticles are 20 nm wide and 60 nm long. Due to their shape, the scattered light is strongly polarized along the particle axis. This can be used to manufacture polarizers for example. Another very interesting application is the use of the polarisation as a nanosensor for orientation, which is a property that is otherwise very hard or even impossible to obtain on a nanometer scale. Thus, we are planning to use such rods to explore single molecules such as molecular motors. The measuring principle is depicted in the picture on the right: the relative orientation is determined by comparing the intensities of the two points. The points are created by light passing through a birefringent crystal. More details can be found in this publication:
Gold Nanorods as Novel Non-Bleaching Plasmon Based Orientation Sensors for Polarized Single Particle Microscopy, Sönnichsen, C., Alivisatos, A.P. Nano Letters 5, 301 (2005)
Gold and silver particles pairs as distance sensors
 Gold and silver particles can be used to determine distances, because the plasmons in two neighboring particles couple and the color changes depend on the distance of the particles. Compared to other sensors based on Förster energy transfers between two dye molecules FRET, the advantage is the indefinite lifetime of the particles that allows for continuous measurements. On the left, you can see the change in color that occurs when two silver particles come close to each other. First, the single particles are blue but as soon as the second particle binds, the pair appears green. Since both the particles and the pair are well below the resolution of an optical microscope, they are seen as a single dot. This work has been published in Nature Biotechnology 23, 741 (2005) .
Gold and silver particles can be used to determine distances, because the plasmons in two neighboring particles couple and the color changes depend on the distance of the particles. Compared to other sensors based on Förster energy transfers between two dye molecules FRET, the advantage is the indefinite lifetime of the particles that allows for continuous measurements. On the left, you can see the change in color that occurs when two silver particles come close to each other. First, the single particles are blue but as soon as the second particle binds, the pair appears green. Since both the particles and the pair are well below the resolution of an optical microscope, they are seen as a single dot. This work has been published in Nature Biotechnology 23, 741 (2005) .
Electron microscopy
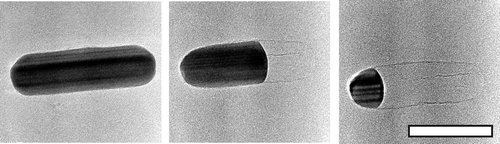 Using in situ transmission electron microscopy, we find that a carbon shell governs the morphological transitionsof gold and silver nanorods upon heating. Encapsulated Ag nanorods show a surprising nonuniform sublimationbehavior starting from one side and leaving behind the shell. Uncovered gold nanorods transform their shapeto spheres well below the bulk melting temperature through surface diffusion, which is prevented by a thincarbon shell.
Using in situ transmission electron microscopy, we find that a carbon shell governs the morphological transitionsof gold and silver nanorods upon heating. Encapsulated Ag nanorods show a surprising nonuniform sublimationbehavior starting from one side and leaving behind the shell. Uncovered gold nanorods transform their shapeto spheres well below the bulk melting temperature through surface diffusion, which is prevented by a thincarbon shell.
'Enhanced thermal stability of gold and silver nanorods through thin surface layers'
Y. Khalavka, C. Ohm, L.Sun, F. Banhart, C. Sönnichsen
Phys. Chem. C 111, 12886 (2007)
